Introduction
The landscape of clinical trials has undergone a rapid and remarkable transformation over the past decade. A glance at the number of registered clinical trials per year reveals a staggering shift from 2000 per year in 2020 to an overwhelming 470,000 in 2023. This exponential growth signifies a landscape that has become increasingly complex, mirroring the surge in published research papers, with nearly 3 million in the last year alone.
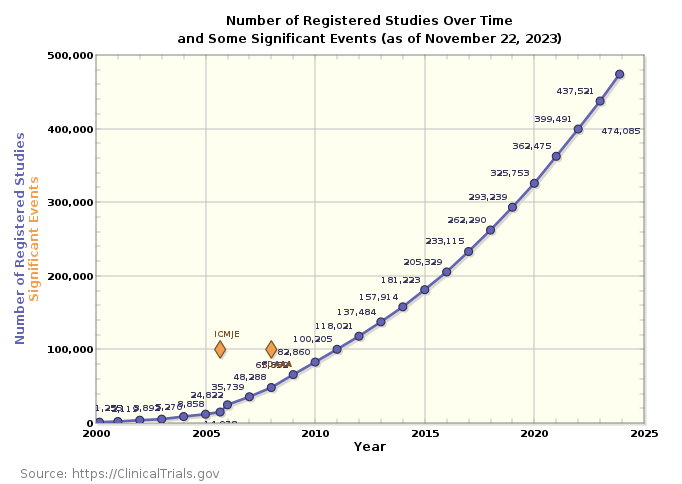
The complexity of contemporary clinical trials results from advancements in medical science, giving rise to intricate protocols and the imperative to operate on a global scale involving diverse patient populations. The global nature of these trials introduces an additional layer of complexity as studies span various geographical regions, each characterized by distinct regulatory nuances, cultural considerations, and healthcare landscapes.
In this evolving landscape, robust management solutions are indispensable. They must not only navigate the intricacies of trial design and execution but also harmonize these diverse aspects across borders. This harmonization ensures consistency, compliance, and ethical conduct, addressing the multifaceted challenges posed by modern clinical trials' global and intricate nature.
This complexity demands meticulous coordination, precise data collection, and seamless collaboration among all clinical trial contributors, from researchers to CROs to funding bodies and other regulatory bodies. As the intricacy grows, so does the necessity for modern solutions, which brings forth a new challenge: Robust management solutions that can scale up with the increase in needs, data, and all the challenges that extensive clinical trials bring. Robust solutions must keep pace and anticipate and address the evolving challenges.
The integration of cutting-edge technologies, artificial intelligence, and advanced analytics becomes imperative to meet the demands of modern clinical research. Electronic Data Capture (EDC) systems ensure the precision of data collection, minimizing the risk of errors and enhancing data accuracy. Advanced analytics and artificial intelligence contribute to efficient trial oversight, swiftly identifying patterns and insights that might elude traditional methods. Collectively, these technological advancements not only streamline the complex web of clinical trial procedures but also significantly contribute to shortening the trial and discovery timeline.
The post below highlights how clinical trials are changing and stresses the crucial role of solid management solutions. The point is simple: it's not just a good idea but a must to use robust management solutions to succeed in this complex time. To do that, we need to pick the right technology and set up the system correctly. When choosing the technology and before opting for the best fit for your study, it is essential to make the difference between software and platforms in the context of clinical trial management and how each contributes to the success of the operations. Software, in this context, represents specialized tools meticulously engineered to cater to specific functions within the clinical trial lifecycle. On the other hand, platforms encompass comprehensive ecosystems that integrate multiple functionalities within a unified framework. They extend their reach across various facets of clinical trial management, from data collection and management to monitoring, reporting, and beyond.
The distinction is akin to the difference between a focused tool with specialized capabilities (software) and a holistic, interconnected system that orchestrates multiple aspects of clinical trial operations (platform). Understanding these distinctions is paramount for researchers and stakeholders, as it lays the foundation for informed decision-making in adopting the most fitting solutions for the intricate challenges posed by modern clinical trials.
Defining the essentials: Software vs. Platforms
In the field of research and clinical trials, it's crucial to establish clear distinctions between software and platforms, as each plays a unique role in the management landscape. The table below displays key criteria and capabilities for each
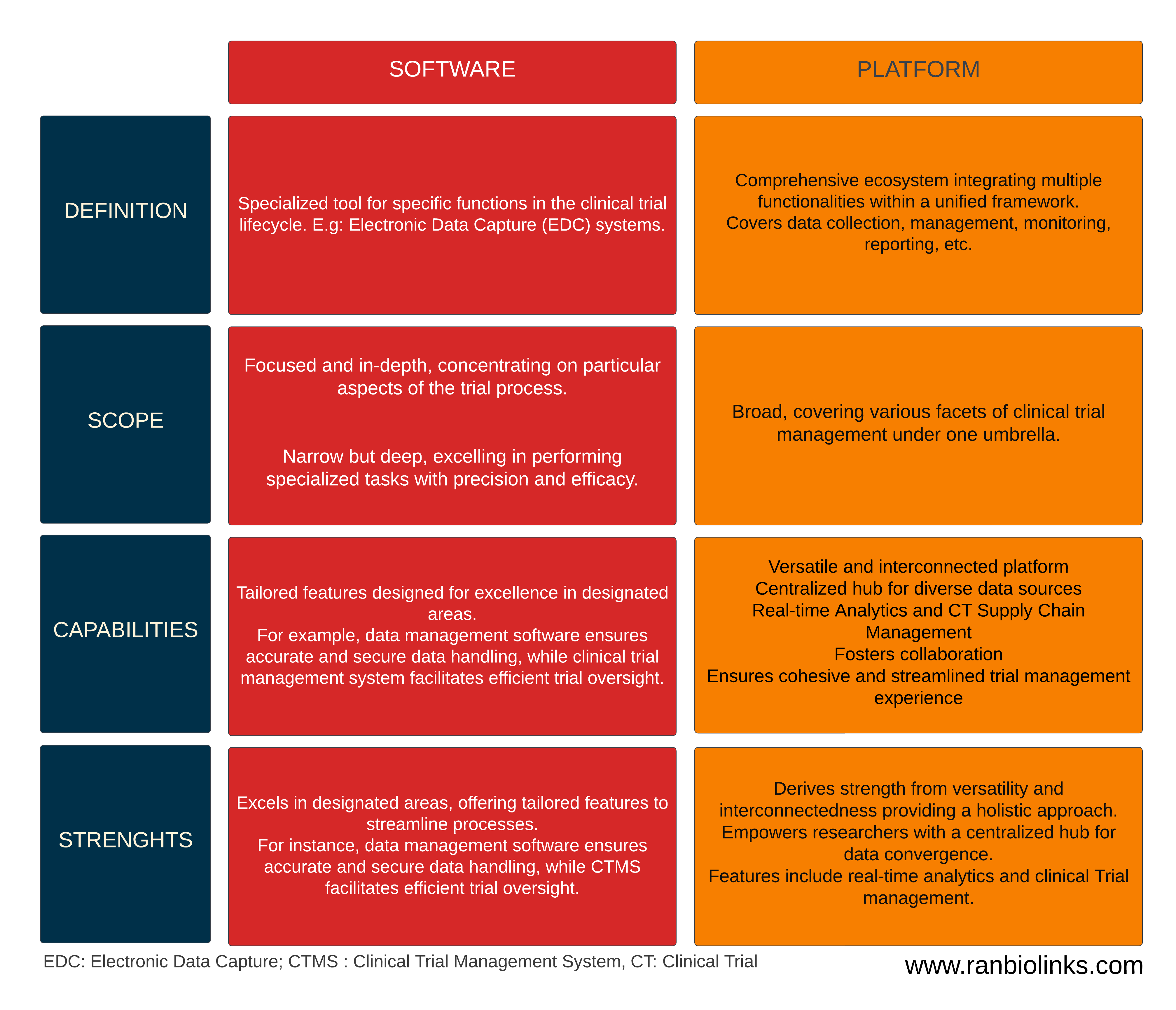
The fundamental difference between software and platforms lies in their focus and breadth. Software excels in specialized tasks, offering depth in a specific area, while platforms provide a comprehensive solution that spans the entire spectrum of clinical trial management. Understanding these distinctions is pivotal in selecting the right tools to meet the unique needs of a particular trial. In the subsequent sections, we'll explore the strengths and considerations associated with each approach, aiding in informed decision-making for effective trial management.
1- The Power of Specialization: Software solutions:
Exploring the benefits of using special tools in clinical trials comes with multiple benefits that significantly enhance the efficiency of clinical trial management. One significant advantage is how these tools focus on specific jobs, like collecting data or organizing information, management, or randomization; they offer precision and expertise in their designated areas. Moreover, the implementation of specialized software tends to be quicker than broader platforms. The specificity of their functions allows for more straightforward integration into existing workflows, requiring less time for training and adaptation.
This swift implementation is particularly crucial in the dynamic landscape of clinical trials, where rapid response and agility are paramount. Thus, specialized software emerges not only as a solution tailored to specific needs but also as a time-efficient asset that contributes to clinical trial operations' overall agility and effectiveness.
While this specialized software presents compelling advantages, it's essential to acknowledge potential drawbacks, and one significant limitation is the potential for limited integration with other trial components. Specialized tools, designed with a specific focus, might operate as standalone entities, leading to challenges in seamless collaboration with broader clinical trial operations. Specialized software, in its targeted functionality, might not inherently possess the flexibility to integrate with these diverse components, creating silos of information. This lack of integration can hinder the holistic visibility needed for comprehensive trial oversight, potentially leading to inefficiencies, data discrepancies, and a fragmented approach to trial management.
2- The Power of Integration: Platform solutions: Platforms in clinical trial management are comprehensive, one-stop solution seamlessly integrating various aspects to provide a holistic and unified approach. Unlike specialized software, platforms act as multifaceted ecosystems that consolidate diverse functionalities within a centralized framework. From data collection and management to monitoring, reporting, and beyond, these platforms serve as a nexus where all components converge. This integration facilitates real-time collaboration, allowing stakeholders to access a consolidated view of the entire trial landscape. Electronic Health Records (EHR), Electronic Data Capture (EDC), and Clinical Trial Management Systems (CTMS) coalesce into a cohesive system, erasing silos and promoting synergy.
Additionally, platforms often offer features like real-time analytics, further solidifying their role as comprehensive solutions catering to the intricate needs of modern clinical trials. For instance, in the complex setting of a large-scale international clinical trial spanning distinct continents, a platform would serve as a centralized hub monitoring diverse regulatory landscapes and varying healthcare infrastructures across continents. This is only possible if proper governance is in place to ensure that the software complies with the different regulatory bodies and maintains ethical standards.
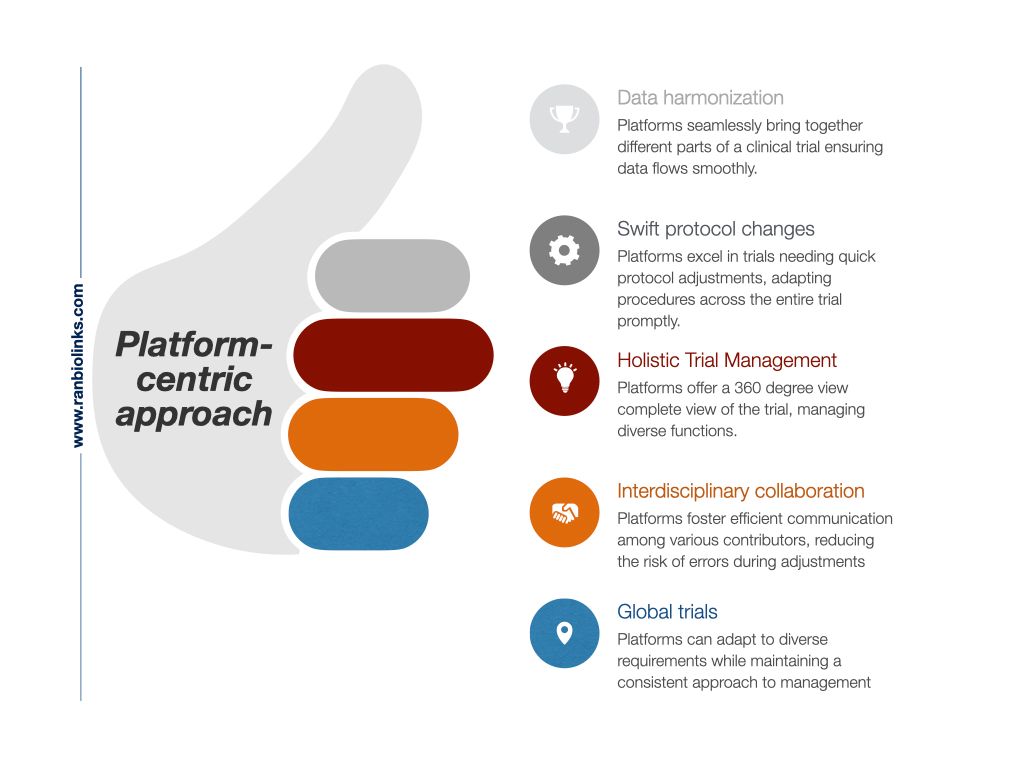
In my opinion, the most vital asset of platforms is interconnectedness, which provides a 360-degree view of the trial, allows for quick adjustments in trial protocols, and allows immediate communication across international teams, ensuring that the trial remains agile in the face of evolving complexities.
Key considerations before choosing a solution for your clinical trials
My takeaway from my experience managing multi-site clinical trials is the recurrent choice of implementing diverse software solutions within the same trial. This tendency is often due to financial constraints or a lack of awareness about alternatives. While these disparate solutions cater to specific needs, they bring forth critical challenges, most notably the formation of data silos, lack of data-driven decisions, lack of agility, and obstacles in attaining a holistic 360-degree view of the trial.
This fragmented approach tends to contribute to the frustration of the clinical team, exacerbating the complexities associated with using disconnected tools. Below, I outline the key issues related to this fragmented strategy:
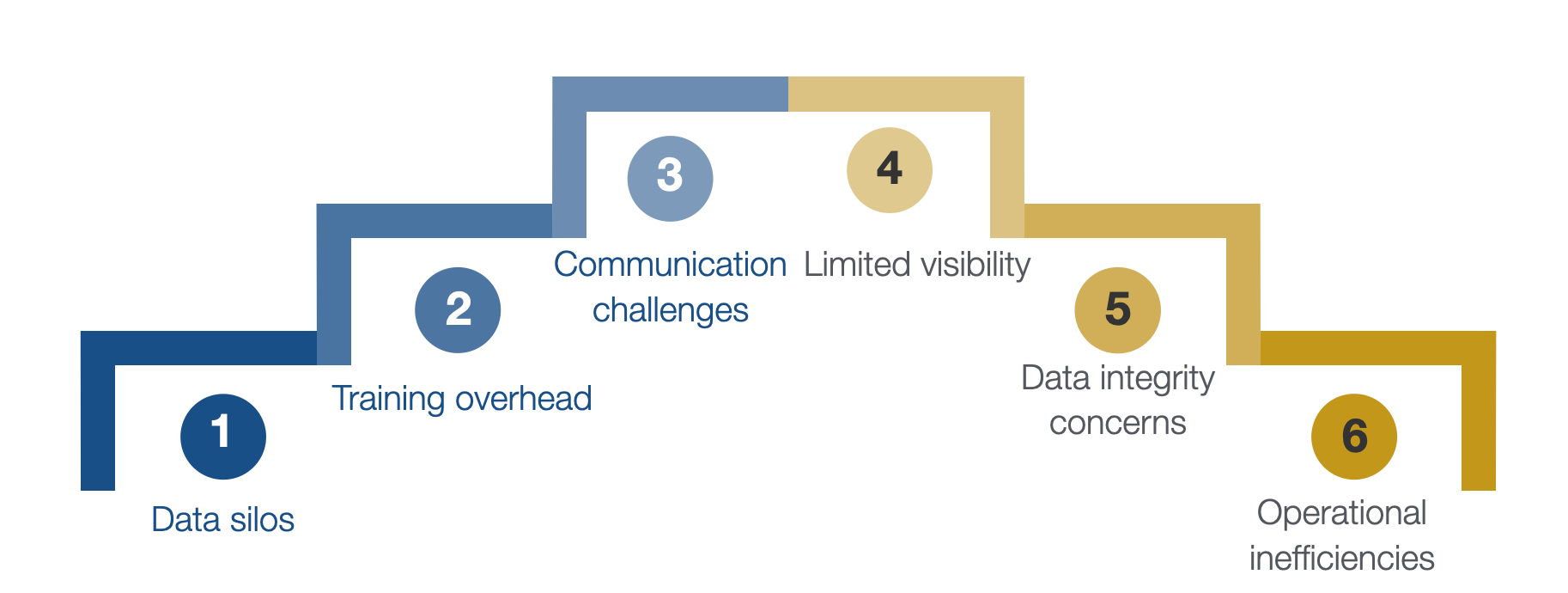
- Data Silos with isolated information: Different software solutions often operate as isolated entities, each managing specific aspects of the trial. This results in the creation of data silos where information is categorized and segregated, hindering the seamless flow of data between different components (e.g., biobanking data, patient de-identified data, laboratory analysis results, etc.)
- Lack of Cohesion with Inconsistent Workflows: Each software solution may follow its workflows and processes, leading to inconsistencies in how data is collected, analyzed, and managed. This lack of cohesion can introduce discrepancies, making it challenging to maintain a standardized approach across the trial.
Adopting a platform-centric approach that integrates various trial components within a unified ecosystem becomes crucial to mitigate these challenges. Platforms offer the advantage of seamless data flow, standardized workflows, and comprehensive oversight, addressing the pitfalls associated with using disparate software solutions in the same clinical trial.
- Limited Visibility: Using different software tools makes achieving a holistic view of the trial difficult. Stakeholders, including researchers, clinicians, and sponsors, might struggle to access a centralized and up-to-date overview of the entire trial landscape.
- Data integrity concerns and risk of errors: Manually transferring data between disparate systems increases the chance of mistakes. Inconsistencies in data entry, duplication, or misinterpretation may arise, compromising the integrity of the trial data. These errors can lead to inaccurate analyses and decision-making.
- Operational inefficiencies due to redundant efforts: Multiple software solutions may result in teams duplicating tasks across different platforms. This redundancy consumes valuable resources and introduces opportunities for inconsistencies and operational inefficiencies.
- Workflow disruption with disjointed processes: Clinical teams often face frustration due to confusing processes caused by different software solutions. Transitioning between disparate tools interrupts the natural flow of work, requiring team members to adapt to varied interfaces and procedures. This disruption can impede efficiency and contribute to frustration among the clinical staff.
- Training Overhead with varied skill requirements: Each software solution may demand unique skills and training. Clinical team members may navigate multiple training programs tailored to a specific tool. This diversity in skill requirements not only consumes valuable time but also adds to the complexity of the trial.
In essence, the frustration experienced by the clinical team is a critical aspect to consider when evaluating the choice between disparate software solutions and integrated platforms. A unified platform approach to streamline workflows and enhance collaboration can significantly alleviate these frustrations, fostering a more efficient and cohesive clinical trial management.
Conclusion
The choice between disparate software solutions and integrated platforms in clinical trial management extends far beyond technical considerations—it significantly impacts the clinical team's efficiency, collaboration, and overall experience. Integrated platforms present a persuasive solution, effectively tackling challenges posed by isolated software solutions, which may offer short-term cost-effectiveness but lack long-term viability. As clinical trials evolve in complexity, the choice between disparate software solutions and integrated platforms becomes pivotal. The focus should be on meeting technical requirements and creating an environment that empowers the clinical team, ultimately contributing to the success and integrity of the trial.
Reference:
https://classic.clinicaltrials.gov/ct2/resources/trends#RegisteredStudiesOverTime



