Introduction
The importance of data in clinical research
Data is the lifeblood of clinical research, driving the discovery of new treatments, therapies, and interventions that improve patient outcomes and advance medical knowledge. Clinical trials generate vast amounts of data, from patient demographics and medical histories to treatment outcomes and adverse events. This data is critical for assessing the safety and efficacy of new drugs, devices, and procedures, and for informing regulatory decisions and clinical practice guidelines. Without high-quality, reliable data, clinical research would be severely hindered, and the development of new medical innovations would be significantly slowed.
The need for robust data governance frameworks
As the volume and complexity of clinical research data continue to grow, so too does the need for robust data governance frameworks. Data governance refers to the policies, procedures, and practices that ensure the integrity, security, and accessibility of data throughout its lifecycle. In the context of clinical research, data governance is essential for ensuring that data is collected, managed, and analyzed in accordance with regulatory requirements, ethical standards, and best practices. Without effective data governance, clinical research data may be compromised by errors, inconsistencies, or breaches, leading to delays, cost overruns, and even patient harm.
The benefits of effective data governance in clinical research
Effective data governance provides numerous benefits to clinical research organizations, sponsors, and patients. By establishing clear roles and responsibilities for data management, data governance frameworks help to ensure that data is collected, stored, and analyzed in a consistent and reliable manner. This, in turn, facilitates the sharing and reuse of data across studies and institutions, accelerating the pace of discovery and reducing the cost and time required to bring new treatments to market. Additionally, by implementing robust security and privacy controls, data governance frameworks help to protect the confidentiality and integrity of patient data, enhancing public trust in clinical research and encouraging greater patient participation in clinical trials.
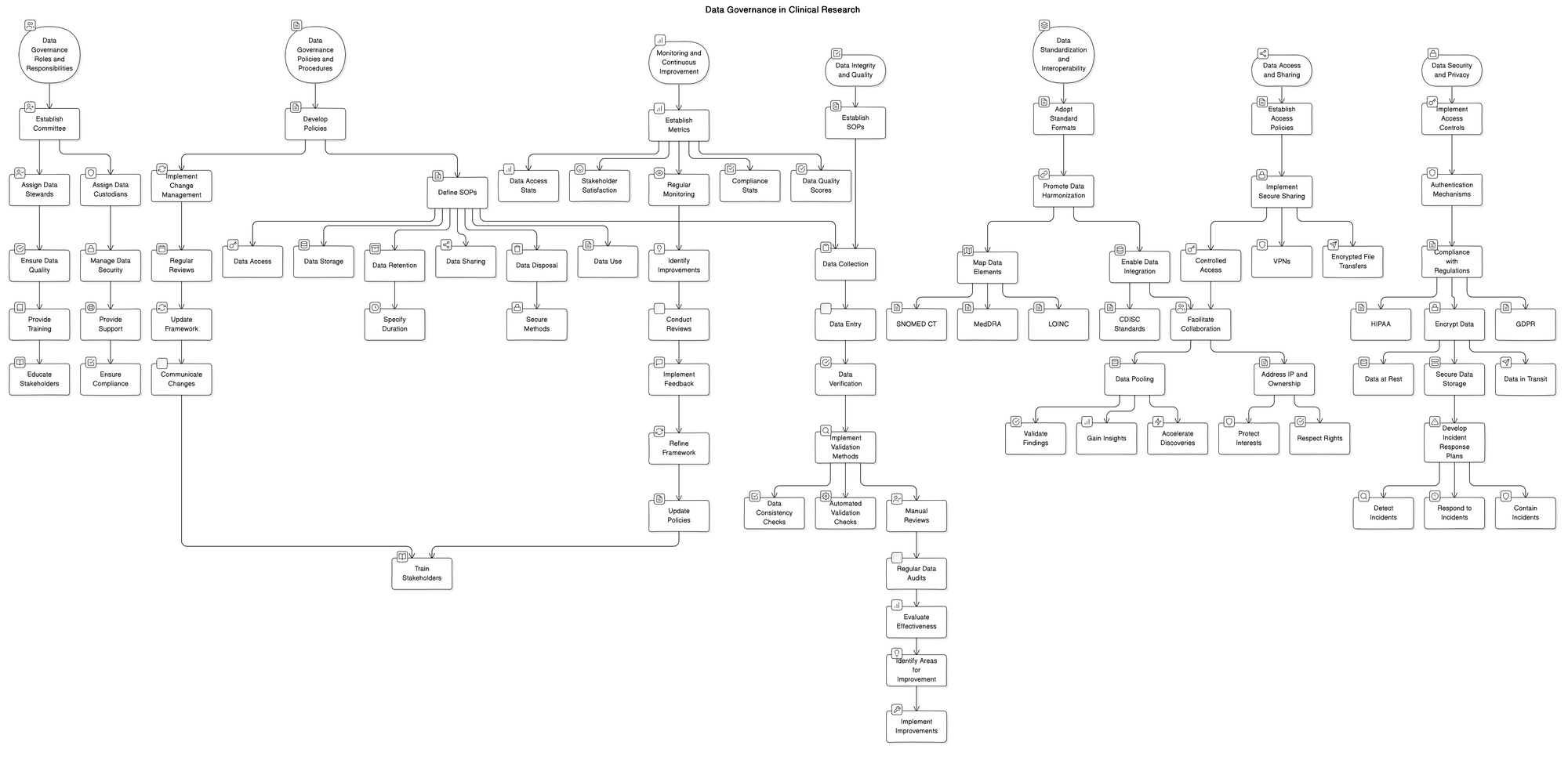
Data Integrity and Quality
Data integrity and quality are foundational elements of a robust data governance framework in clinical research. To ensure the accuracy and completeness of data, organizations must establish comprehensive data quality control processes. These processes should include well-defined standard operating procedures (SOPs) that outline the steps for data collection, entry, and verification. Implementing data validation and reconciliation methods is crucial to identify and resolve any discrepancies or errors in the data. This can be achieved through automated validation checks, data consistency checks, and manual reviews by trained personnel. Regular data audits and assessments should be conducted to evaluate the effectiveness of the data quality control processes and identify areas for improvement. These audits may involve sampling and reviewing data sets, examining data entry logs, and assessing compliance with SOPs. By prioritizing data integrity and quality, clinical research organizations can have confidence in the reliability and trustworthiness of their data, enabling them to make informed decisions and draw valid conclusions from their research findings.
Data Security and Privacy
In the realm of clinical research, safeguarding sensitive patient information is of utmost importance. Robust data governance frameworks must prioritize the implementation of stringent access controls and authentication mechanisms to ensure that only authorized personnel can access confidential data. Compliance with data protection regulations, such as HIPAA in the United States and GDPR in the European Union, is non-negotiable. Healthcare organizations must meticulously adhere to these regulations to maintain the trust of patients and avoid costly penalties. To further fortify data security, utilizing encryption techniques and secure data storage solutions is essential. Encrypting data both at rest and in transit adds an extra layer of protection, making it virtually impossible for unauthorized individuals to decipher sensitive information. Moreover, developing comprehensive incident response plans is crucial to mitigate the impact of potential data breaches. These plans should outline clear protocols for detecting, containing, and responding to security incidents, minimizing the risk of data exposure and reputational damage. By prioritizing data security and privacy, clinical research organizations can foster a culture of trust and integrity, ultimately empowering groundbreaking discoveries while upholding the highest standards of patient confidentiality.
Data Standardization and Interoperability
In the realm of clinical research, data standardization and interoperability play a pivotal role in ensuring the efficiency, accuracy, and reproducibility of research findings. Adopting industry-standard data formats and terminologies is essential to maintain consistency and facilitate seamless communication among researchers, healthcare providers, and regulatory bodies. By promoting data harmonization across different systems and platforms, organizations can break down silos and enable the smooth exchange of information. This harmonization effort involves mapping data elements to common vocabularies, such as SNOMED CT, LOINC, and MedDRA, which provide a unified language for describing medical concepts, laboratory tests, and adverse events. Enabling seamless data integration and exchange is crucial for leveraging the full potential of clinical research data. Standardized data formats, such as CDISC (Clinical Data Interchange Standards Consortium) standards, ensure that data collected from various sources can be easily combined, analyzed, and shared among stakeholders. Moreover, leveraging data standards facilitates collaboration and data pooling, allowing researchers to access larger and more diverse datasets. By pooling data from multiple studies or institutions, researchers can gain deeper insights, validate findings, and accelerate the discovery of new treatments. Implementing robust data governance frameworks that prioritize data standardization and interoperability is essential for driving innovation, enhancing patient outcomes, and advancing the field of clinical research.
Data Access and Sharing
In the realm of clinical research, effective data access and sharing play a pivotal role in fostering collaboration, accelerating scientific discoveries, and ultimately improving patient outcomes. To achieve these goals, organizations must establish clear data access policies and procedures that strike a balance between data security and accessibility. Implementing secure data sharing mechanisms, such as encrypted file transfers and virtual private networks (VPNs), ensures that sensitive information remains protected while enabling authorized stakeholders to access the data they need. Facilitating controlled access to data is crucial, as it allows research teams to collaborate and build upon each other's findings without compromising patient privacy or data integrity. By encouraging data sharing and collaboration among research teams, organizations can break down silos and promote a culture of openness and innovation. However, it is equally important to address intellectual property and data ownership considerations, ensuring that the rights and interests of all parties involved are respected and protected. By navigating these complexities and implementing robust data governance frameworks, clinical research organizations can unlock the full potential of their data assets and drive meaningful advancements in healthcare.
Data Governance Roles and Responsibilities
In order to effectively implement a data governance framework within a clinical research organization, it is crucial to clearly define the roles and responsibilities of all stakeholders involved. This process begins with the establishment of a data governance committee or council, which is responsible for overseeing the development, implementation, and ongoing maintenance of the framework. The committee should consist of representatives from various departments, including IT, clinical operations, regulatory affairs, and data management, to ensure a comprehensive approach to data governance. Once the committee is in place, the next step is to assign data stewards and data custodians. Data stewards are responsible for ensuring the quality, accuracy, and consistency of data within their designated domain, while data custodians are tasked with the technical management and security of the data. These roles are critical in maintaining the integrity and usability of clinical research data throughout its lifecycle. To support the success of the data governance framework, it is essential to provide comprehensive training and support to all stakeholders. This includes educating them on the importance of data governance, their specific roles and responsibilities, and the processes and tools available to them. By investing in the development of a strong data governance team and providing them with the necessary resources, clinical research organizations can ensure the long-term success of their data governance initiatives.
Data Governance Policies and Procedures
To ensure the integrity, security, and efficiency of clinical research data, it is crucial to establish a robust data governance framework. This framework should encompass comprehensive policies and standard operating procedures (SOPs) that guide data management practices throughout the research lifecycle. Developing clear data governance policies is the foundation of this framework, outlining the principles, roles, and responsibilities for data stewardship. These policies should cover data collection, storage, access, sharing, and use, ensuring compliance with regulatory requirements and ethical standards. Additionally, SOPs should be established to provide step-by-step instructions for various data management tasks, such as data entry, validation, backup, and archiving. These SOPs ensure consistency and accuracy in data handling across different research teams and studies. Furthermore, data retention and disposal guidelines should be defined to specify the duration for which research data must be retained and the secure methods for disposing of data when no longer needed. This helps to minimize data privacy risks and ensures compliance with data protection regulations. Lastly, implementing change management processes is essential to keep data governance policies and procedures up to date with evolving regulatory requirements, technological advancements, and best practices in clinical research. Regular reviews and updates of the data governance framework should be conducted, with clear communication and training provided to all stakeholders to ensure adherence to the latest policies and procedures.
Monitoring and Continuous Improvement
A robust data governance framework is not a one-time implementation but an ongoing process that requires continuous monitoring, evaluation, and improvement. To ensure the effectiveness of data governance practices in clinical research, it is crucial to establish metrics and key performance indicators (KPIs) that align with the organization's goals and objectives. These metrics may include data quality scores, data access and usage statistics, compliance with regulatory requirements, and stakeholder satisfaction levels. Regular monitoring of these KPIs enables the identification of areas for improvement and helps measure the success of data governance initiatives. Conducting periodic reviews and audits of data governance practices is essential to maintain accountability, identify gaps, and ensure adherence to established policies and procedures. Furthermore, implementing feedback mechanisms, such as surveys and focus groups, allows stakeholders to provide valuable input and suggestions for enhancing data governance processes. By actively seeking and incorporating stakeholder feedback, organizations can foster a culture of collaboration and continuous improvement. Lessons learned from monitoring, audits, and feedback should be used to refine and update data governance frameworks, ensuring they remain relevant, effective, and adaptable to the evolving needs of clinical research.
Conclusion
Data governance is the cornerstone of successful clinical research, enabling organizations to efficiently manage, protect, and utilize their valuable data assets. By implementing robust data governance frameworks, research institutions can ensure data integrity, security, and compliance with regulatory requirements. This, in turn, fosters an environment conducive to innovation and groundbreaking discoveries in patient care and treatment development. The impact of effective data governance extends far beyond the confines of research facilities, as it ultimately contributes to improved patient outcomes and advances in medical science. Organizations that prioritize data governance are better equipped to collaborate with partners, share knowledge, and drive meaningful progress in the field of clinical research. It is imperative that research institutions recognize the critical role of data governance and take proactive steps to establish and maintain comprehensive data governance strategies. By doing so, they will unlock the full potential of their data, accelerate scientific breakthroughs, and pave the way for a brighter, healthier future for patients worldwide.




