1. Introduction
In today's world of healthcare and research, biobanking isn't just a place to keep biological samples. It's like a guardian, ensuring everything follows the rules and procedures exactly. It's not just about collecting and storing samples; biobanking is like a watchman, making sure all the information is not just a lot but also carefully controlled. The quest for an efficient tracking system does not end with a top-of-the-line barcode scanner or a highly-developed tracking software. In this blog post, we'll explore the layers that makes up the heart of biobanking, looking at how organizing information and doing things just right come together to shape the future of clinical research. Nowadays, cloud-based platforms and innovative data management solutions are redefining traditional tracking in biobanking. I will show their potential to transform conventional biobanking practices, taking data integrity and sample tracking to new heights.
The quest for an efficacious tracking system does not end with a top-of-the-line barcode scanner or a highly-developed tracking software.
2. The evolution of tracking technologies: Recall of traditional and modern tracking Methods
The significance of accurate tracking methods for biobanking cannot be overstated. Most of the traditional methodologies for tracking bio-samples involved manual procedures. These included hand-written logs, physical labels, and ledger formats to document sample collection, storage, and movements. Archiving the data was typically achieved through rudimentary database software or hard-copy filing systems. Records were queried manually and required significant time and effort to extract meaningful information or to trace errors. While these methods were invaluable at their time, their shortcomings were apparent in light of the increasing scale and complexity of bio-samples being handled in clinical research today. Data tracing in traditional systems also came with errors, inaccuracies, and inefficiencies.
Later, things have changed considerably with the arrival of modern tracking technologies. A typical modern tracking procedure incorporates barcode, Computerized Maintenance Management Systems (CMMS), Laboratory Information Management Systems (LIMS), and other technological methods. This technology permits researchers to track and document specimen data in real time, drastically reducing human error, time, and labor costs associated with traditional tracking methods. Barcoding systems allow users to label, identify, and trace thousands of samples with minimal effort and negligible risk of human error. Similarly, LIMS and CMMS offer sophisticated platforms for managing warehouse operations, controlling inventory, managing schedules, and ensuring quality control. The rapid propagation of cutting-edge technologies in biobanking has led to enormous advancements in the depth and breadth of tracking methods. Advances such as high connectivity, cloud computing, big data analytics, and artificial intelligence are redefining tracking processes in the biobanking sphere. They enable the interconnectedness of all biobanking devices and allow seamless flow of information, thus facilitating real-time tracking.
In tandem, cloud computing aids in housing vast amounts of data from these devices, ensuring their retrieval and usage become much more straightforward. Big data analytics trends have complemented these developments and taken sample handling to an unprecedented level, making it easier to analyze complex data, find patterns, and optimize processes in biobanking. Concurrently, machine learning algorithms and artificial intelligence have revolutionized error detection and analysis within biobanking databases, automating error diagnosis, identifying inconsistency patterns, and suggesting corrective measures. In essence, these technological advancements present a paradigm shift; they offer more reliable, efficient, and sophisticated tracking solutions for biobanking, immensely enhancing data integrity in sample handling.
3. Benefits and potential downsides of redefined modern tracking practices in biobanking
Ensuring precision in the handling, storing, and retrieving of bio-samples is a top priority in the field, as it guarantees the optimal integrity of these invaluable resources. Modernized tracking practices, utilizing advanced technologies such as barcode systems and RFID, are pivotal in enhancing accuracy by enabling real-time monitoring and reducing manual errors. Apart from elevating accuracy, these redefined tracking systems significantly improve operational efficiency by automating tasks, minimizing misplacements, and accelerating sample access. This not only streamlines biobanking workflows but also frees up staff to focus on core research activities. The trust in biobanking data is bolstered through improved reliability, transparency, and reduced error rates, fostering stronger collaborations between biobanks and researchers. While implementing these advanced tracking solutions involves initial costs, the long-term benefits, including decreased manual errors, time savings, and increased sample longevity, make it a cost-effective strategy.
Moreover, tracking in biobanking serves as a vital tool in ensuring regulatory compliance. Regulatory bodies extensively emphasize the need for detailed records regarding the storage, usage, and disposal of samples. Therefore, the possession of an effective tracking system not only ensures operational ease but safeguards from potential legal ramifications.
The importance of data integrity in biobanking cannot be overstated, as it directly influences the quality, reliability, and value of biological samples, playing a crucial role in advancing scientific discoveries and therapeutic developments. Let's take a quick look at the advantages and challenges introduced by the adoption of modern platforms in the realm of biobanking:

4. Mastermind Your Samples: Are you the maestro of tracking and lineage?
Being the mastermind of your samples involves more than just keeping things organized; it's about taking charge of the entire process. You're not just keeping an eye on where they are, but you're also the one ensuring that their full history is well-documented. It's like telling the life story of each sample. Imagine you're keeping a family tree, but instead of people, it's about where each sample comes from, where it's been, and how it's been treated. This lineage is super important because it adds a layer of trust and reliability to the whole process. When you know the history of a sample, you can be more sure that the data and results you get from it are accurate. So, we're not just managing samples; we're also telling their stories, ensuring every step is recorded and understood, thanks to the combination of technology and human expertise.
A well-managed biobank relies on the indispensable contribution of skilled managers and teams who serve as the backbone of the entire operation. These experts play a pivotal role in upholding stringent standards and meticulously adhering to established protocols, ensuring the reliability and accuracy of the stored biological samples. It's not just about the machines; it's about the experienced hands guiding the process. Technology helps in making things move faster and more precisely, but it's the people who use their judgment and experience to make sure everything is top-notch. It's like having a great team where everyone has their role, and together, they make sure every decision fits the main goal: keeping the quality and reliability of biobank samples in check.
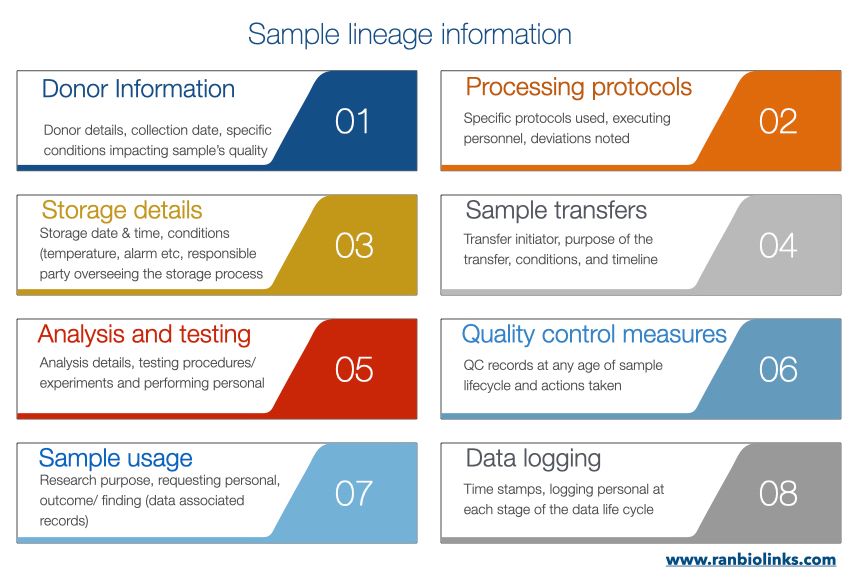
The flowchart below illustrates the meticulous documentation process for tracking the lineage of biological samples in a biobanking setting. From initial donor information and processing protocols to storage details, sample transfers to usage in analyses where each step is carefully recorded. The chart emphasizes the importance of maintaining quality and reliability through quality control measures, providing transparency and traceability. Including data logging with timestamps ensures a detailed record of the sample's journey, not only safeguards the integrity of research outcomes but also serves as a valuable resource for future investigations and collaborations.
5. Orchestrating Information - The role of data in biobanking
Striving for data integrity necessitates the adoption of tracking protocols that are immune to human error, thereby preserving the sanctity of the biobank’s data. The need for structured and organized data plays a pivotal role and is the backbone of biobanking operations. Without a well-organized data structure, the vast repository of biological samples becomes a chaotic ensemble, affecting the seamless execution of research protocols and compromising the reliability of results. Establishing a structured and organized data structure in biobanking requires careful planning, adherence to industry standards, and the integration of best practices. Here are the key requirements for achieving a robust data structure:
1- Data Standardization:
- Define and adhere to standardized formats for data entry, ensuring consistency across all records.
- Implement standardized coding systems for variables, minimizing ambiguity and facilitating data integration.
2- Metadata Definition:
- Clearly define metadata, including essential information about each sample, such as donor details, collection methods, and processing protocols.
- Establish a comprehensive metadata dictionary that outlines the structure and meaning of each data element.
3- Unique Identifiers:
- Assign unique identifiers to each sample to ensure traceability and avoid duplication.
- Implement barcode systems, RFID, or other unique identification technologies for efficient sample tracking.
4- Comprehensive Data Dictionary:
- Develop a comprehensive data dictionary that documents all data elements, their definitions, and permissible values.
- Include information on data types, units of measurement, and any specific conventions used in data representation.
5- Data validation and quality control:
- Implement data validation checks to ensure accuracy, completeness, and adherence to predefined standards.
- Conduct regular quality control assessments to identify and rectify any discrepancies in the data. This digital democratization of data provides the benefit of remote monitoring and the availability of real-time updates on sample status.
6- Data security and access controls:
- Implement robust data security measures to protect sensitive information, including donor details and research outcomes.
- Establish access controls to regulate who can view, modify, or delete specific data elements, ensuring data integrity and privacy. Biobanks can upload their inventory to the cloud, where it can be accessed at any time by authorized personnel, integrating accessibility with security.
7- Audit trails:
- Maintain detailed audit trails that log any changes made to the data, including who made the changes and when.
- Ensure the audit trail is accessible for review and compliance purposes.
8- Data integration capabilities:
- Design the data structure to allow seamless integration with other systems and databases, fostering interoperability.
- Ensure compatibility with data exchange standards to facilitate collaboration and data sharing.
9- Scalability and flexibility:
- Design the data structure to be scalable, accommodating future growth in the volume of samples and data.
- Ensure flexibility to adapt to evolving research requirements and technological advancements.
10- Documentation and training:
- Provide thorough documentation on the data structure, including guidelines, procedures, and definitions.
- Conduct regular training sessions for staff involved in data entry and management to ensure consistent adherence to data standards.
By addressing these requirements, a biobanking facility can establish a structured and organized data structure that not only meets current needs but also sets the foundation for robust and efficient data management in the future. Moreover, biobanks should invest in secure and reliable digital defense systems to ensure the safety and security of their digital archives. This could involve using firewalls, strong encryption, and regular security audits to identify potential vulnerabilities and address them promptly. There should also be regular backups of data to off-site storage to safeguard against data loss or corruption. Ensuring that the data collected is accurate and consistent is crucial, and it is possible when implementing robust QA/QC (Quality Assurance/Quality Control) protocols and ensuring the equipment and systems used for data collection are maintained adequately to avoid errors. Lastly, biobanks should have contingency plans to handle disruptions in electricity and internet connectivity, especially in regions prone to power outages. By taking these steps, biobanks can ensure superior data integrity.
6. How to Implement Modern Tracking Techniques in your Lab?
A successful tracking system is not just about the initial push for implementation, but about the continuous effort to guarantee that the system remains productive and efficient.
6.1- Initial steps for the implementation
- Processes assessment: The implementation of modern tracking techniques in the laboratory begins with the assessment of current processes. There should be a comprehensive examination of existing protocols concerning sample handling, storage, and data recording. This baseline information is vital as it dictates what changes, replacements, or improvements should be made.
- Choice of a suitable tracking system: Here, the selection of the right tracking system comes into place. This decision should be influenced not only by the size and type of lab involved but also by the nature of the biobanking tasks to be performed. Consequently, the process includes ensuring the selected system covers most, if not all, needs of the lab. In essence, this implies a system that is precise, detailed, user-friendly, responsive, and flexible enough to handle future growth or changes. After this, the system will require seamless integration into existing processes. This includes training staff, conducting mock runs, and addressing with any technical glitches. Indeed, the initial steps of implementing modern tracking techniques are crucial.
- Identification of the key tracking metrics: Here comes into action the strategic selection of Key Performance Indicators (KPIs) and metrics ideally as part of the study design. These indicators serve as the compass guiding the effectiveness and efficiency of the implemented processes in place and should be integrated into the tracking system. The choice of KPIs should align with the overarching goals of the lab and biobanking initiatives. Examples of relevant KPIs may include turnaround times for sample retrieval, workload monitoring, accuracy in data entry, and the frequency of deviations from established protocols. These can be displayed in the tracking system software itself or a dashboard linked to the biobank database outside of the platform. Metrics are complementary in providing granular insights into specific aspects of the biobanking processes. These could range from the success rates of sample analysis to the frequency of system downtimes or technical issues. Importantly, the selection of KPIs and metrics should be tailored to the unique needs and objectives of the lab, ensuring that they directly contribute to the enhancement of processes, data accuracy, and overall efficiency.
6.2- Potential challenges in adapting modern biobanking systems and possible solutions
Like any other technological implementation, integrating modern tracking techniques might not come without challenges. The most common obstacle is resistance to change. Many times, lab technicians resist the adoption of new systems due to unfamiliarity or the inability to see value in adjusting their methodologies. Other anticipated blocks arise from sensor malfunctions or system failure, which can lead to inaccurate tracking or loss of data. Downtime during the integration phase can also disrupt a facility’s normal operations. Moreover, budget constraints can hinder the acquisition of highly effective systems. Indeed, acknowledging these potential hurdles early and coming up with effective mitigation strategies is critical.
Regardless of the type of lab or the complexity of the tracking system, the challenges encountered are surmountable. For one, addressing resistance to change can be achieved through training. Regular and comprehensive training sessions ensure the lab team understands how to use the new tracking system effectively and, more importantly, "WHY" opting for a given system and not another. Encouraging staff involvement in the selection and implementation process can also enhance acceptance and dedication. Concerning technical problems or system failures, having a reliable IT and data support team at the ready is crucial. This team should be equipped to tackle any arising issues, thereby minimizing downtime and data loss. For budget constraints, one effective strategy is to prioritize tracking needs in line with the available resources and propose this as an essential piece in grant budget proposals.
6.3- Tips for successful execution and maintenance
The successful execution and maintenance of any implemented tracking system in your lab depend heavily on several principles. Firstly, continuous training is important. This ensures lab staff stay updated on new features and can maximize the potential of the tracking system. In regards to minimizing system failures, preventive maintenance cannot be overstated. Regular check-ups and software upgrades can detect potential problems early, fostering a proactive approach rather than a reactive one. Lastly, it is advisable to have an efficient backup strategy. This includes having an off-site backup of all the data collected as well as a backup tracking method to use if the central system fails without notice. A successful tracking system is not just about the initial push for implementation but the continuous effort to guarantee that the system remains productive and efficient.
7. Conclusions
Starting a biobank is like putting together a well-thought-out plan. It involves looking at many things, like figuring out if it's doable, having qualified personnel who understand the how and why of the entire process, and making sure it is well executed. A crucial part of this plan is choosing the right way to keep track of everything.
It's not just about picking software; it's about creating a smart strategy. This strategy connects information, rules, ways of doing things, and the skills of the people involved. Think of it like organizing a perfect team that works smoothly. This blog has talked about the importance of organized data, how people and technology need to work together, and how to pick the right goals to measure success.
A world where millions of curated samples stand secure with immaculate data integrity is not beyond our reach. More than a hopeful expectation, it is feasible given all the progress we witness in modern tracking technologies. Biobanking institutions are at the edge of an era where real-time acquisition, maintenance, and analysis of the data collected while maintaining data integrity is possible. The beckoning future, however, demands adaptability and willingness to embrace these technologies among research institutions.
But let's not forget the big idea biobanking is about allowing an open and reliable source of samples and data to be widely used to push science and medicine forward. So, it's the smart plan that shapes the future of biological research.
References and additional resources
https://www.biobanking.com/top-lims-softwares/
https://link.springer.com/article/10.1007/s00428-021-03151-0
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3409343/
https://www.technologynetworks.com/immunology/white-papers/best-practices-for-biobanks-and-biorepositories-357536
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9275522/



